The Department of Health requires suspected cases of measles to be notified immediately without waiting for laboratory confirmation.
Measles is an urgent, highly contagious, notifiable disease. Secondary infections occur in 75-90% of susceptible household contacts
Transmission of the measles virus is by respiratory droplets and direct contact with respiratory secretions
Serological testing and PCR are the mainstays of laboratory diagnosis
Background
Measles is a highly contagious disease with secondary infections occurring in 75 – 90% of susceptible household contacts.
1
With suboptimal vaccination coverage in some areas, measles outbreaks remain an unfortunate reality in Australia.
2
A single case therefore has significant public health implications.
Clinical features
Transmission of measles virus is by respiratory droplets and direct contact with respiratory secretions. The virus can also survive on inanimate objects in the patient’s environment for at least 30 minutes.
After an incubation period of 10 days (range 7 – 18 days) patients develop a prodrome consisting of fever, malaise, cough, coryza and non-purulent conjunctivitis.
Koplik’s spots may develop during this time. These are whitish spots on an erythematous background on the buccal mucosa classically arising opposite the molar teeth.
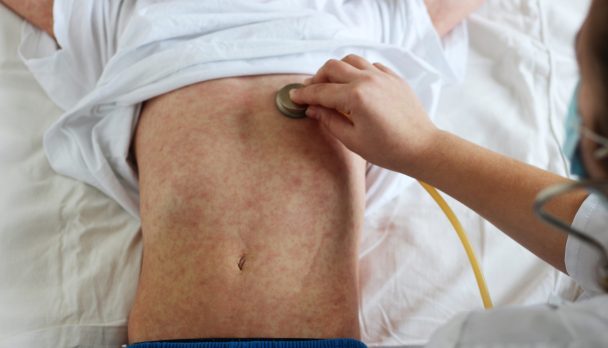
After about four days, a morbilliform rash appears, initially on the face and head, then extending to the trunk and limbs (Figure 1). The rash lasts 3 – 7 days.
Patients usually make a full recovery, but complications including otitis media, pneumonia, seizures, and rarely encephalitis (subacute sclerosing panencephalitis) can occur.
The case fatality rate in stable populations is estimated at around 2%, but rates up to 32% have been seen in refugee and displaced populations.
4
Laboratory testing
Serological testing and PCR are the mainstays of laboratory diagnosis.
In the early stages of infection, a single serology result demonstrating negative measles IgG and positive IgM in the context of the clinical picture outlined above provides strong evidence for a case of measles.
It is important to note that serology can be negative in the early stages of infection. In a minority of patients, IgM may not be detected up to four days after the rash onset.
5
Definitive serological diagnosis can be established with acute and convalescent sera, usually taken 10 – 14 days apart. A diagnostic rise in measles-specific IgG is a reliable indicator of recent infection.
In early infection, PCR is performed on nose and throat swabs.
Nose and throat swabs are usually pooled and analysed together in the testing laboratory.
Swabs sent in viral or universal transport media are acceptable for testing, as are ‘dry’ swabs (no transport media). Swabs using bacterial transport media should be avoided as rates of viral detection may be lower.
Other specimens that can be used for PCR are first pass urine and anticoagulated blood.
When positive, PCR provides rapid confirmation of the clinical picture.
Case definition for measles
| Initial investigation of suspected Measles |
| ▪ Generalised maculopapular rash usually lasting three or more days
and
▪ Fever (at least 38° if measured) present at the time of rash onset
and
▪ Cough, coryza, conjunctivitis and Koplik’s spots |


| Laboratory Testing – Serology (IgG & IgM) and PCR. |
Treatment and prevention
Treatment of measles remains supportive only.
Infection control measures are important in order to avoid secondary cases.
In the clinic;
- The receptionist receiving patients should be alert to possible measles cases.
- Those presenting with fever and rash should be given a single use mask and isolated from other patients
- Consultation rooms used for assessment of suspected measles cases should be left vacant for at least 30 minutes after the consultation.3
Measles vaccination as part of the routine immunisation schedule for clinic patients is of the utmost importance.
It is also important is ensuring that clinic staff vaccinations are kept up-to-date.
Notification
Due to its public health importance, the Department of Health requires all suspected cases of measles to be notified immediately without waiting for laboratory confirmation. This will help facilitate timely follow-up of the contacts, vaccination where required and will help prevent further transmission of the virus.
References
- Perry RT, Halsey NA. The Clinical Significance of Measles: A Review. J Infect Dis. 2004 May 1; 189(S1): S4-16. DOI: 10.1086/377712
- Victorian Department of Health, Blue Book, Infectious Diseases Epidemiology and Surveillance, Measles. [Accessed 8.9.2014] Available at: https://www2.health.vic.gov.au/public-health/infectious-diseases/disease-information-advice/measles
- Kouadio IK, Kamigaki T, Oshitani H. Measles outbreaks in displaced populations: a review of transmission, morbidity and mortality associated factors. BMC Int Health Hum Rights. 2010 Mar 19; 10: 5 [Accessed 8.9.2014]. DOI: 10.1186/1472-698X-10-5
- General Practice Pathology is a regular column each authored by an Australian expert pathologist on a topic of particular relevance and interest to practising GPs. The authors provide this editorial free of charge as part of an educational initiative developed and coordinated by Sonic Pathology.