Articles / Botox and fillers to come under greater scrutiny by the medical regulator. Will it be too little too late?

The Australian Health Practitioner Regulation Agency (AHPRA) has announced it will expand its “crackdown” on the cosmetic surgery industry. As the agency responsible for registering, accrediting and disciplining health practitioners, AHPRA is well placed to reshape conduct in what sociologists once called the appearance industry.
It plans to develop stricter guidelines for non-surgical cosmetic procedures – especially advertising practices, consent procedures and pre-procedure suitability screening.
The regulator’s primary targets are health practitioners authorised to prescribe restricted drugs (schedule 4 medicines) to patients for aesthetic enhancement. The compounds are regulated differently across each state and territory, but they are generally restricted to medical and nursing practitioners or people directly supervised by them.
But how does the regulator propose to make aesthetic medicine safer for patients, and what problems might lie beyond its reach?
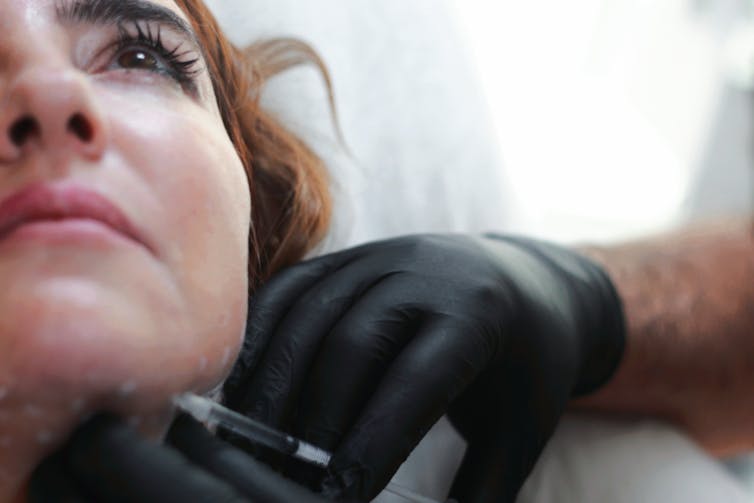
AHPRA’s statement sets out a range of treatments it hopes to make safer for patients. Among them is the most common cosmetic procedure in the world: botulinum toxin type A, commonly known as Botox.
Widely used to reduce wrinkles, Botox is also the most potent neurotoxin ever discovered and was first proposed as a chemical weapon. It’s also injected to treat eyelid spasms, excessive sweating, some bladder disorders and migraine.
Botox functions only at extremely low doses and has been approved for market supply since the early 2000s. Research demonstrates it is safe and effective for cosmetic purposes. But a comprehensive study from the United Kingdom found about 16% of patients reported adverse events including bruising, headache and paresis (muscle weakness or paralysis).
One critical aspect of its safety is the injector’s skill. The Nursing and Midwifery Board of Australia recently updated its position statement on cosmetic injections, underlining the skills expected.
The other injectables to be reviewed – dermal fillers, such as the widely used hyaluronic acid and fat-dissolving agents like deoxycholic acid – present their own safety issues. The death of a patient given fillers in Sydney in 2017 drew attention to the risks of these treatments and the importance of oversight.
As AHPRA acknowledges, a different kind of risk is presented by the connection between Botox and body image disorders. One recent study found recipients of Botox reported fewer body dysmorphic symptoms than a control group. Others have shown patients with histories of body image distress tend to express dissatisfaction with cosmetic procedures and their mental health may worsen after treatment.
But the prospect of a patient with body dysmorphia also raises important legal questions about medical consent and the practitioner’s duty to warn a patient of relevant harms.
The Australian legal standard of medical consent, established in 1991, requires a practitioner to warn the patient of all “material” risks – those that the specific patient does or would consider significant – posed by the treatment or procedure. The same principle was adopted by the English courts in 2015.
For patients with mental health issues, a practitioner would usually identify the likelihood of increased mental distress as a material risk. Valid consent would therefore entail discussion of the psychological risks of treatment.
The review will also home in on the risks of “thread lifting”, where barbed absorbable sutures are threaded under the skin to pull it into a certain position, and intense pulsed light (IPL) treatments for scar resurfacing and hair removal.
Manipulative media environments can exacerbate patients’ vulnerability.
While some doctors may seek to capitalise on insecurity, other promotions might normalise interventions and understate the risks.
COVID lockdowns and the closure of clinics reportedly increased stress for patients seeking cosmetic procedures. Others expressed increased concern about their facial appearance due to the apparently unflattering light of teleconferencing.
A recent study of Tiktok videos tagged with #dermalfillers found most videos, many of which were promotions, lacked any reference to the risks of treatments.
AHPRA proposes to address these issues by reformulating the guidelines for advertising, introducing stricter rules to control “before and after” images, social media influencing and reinforcing the ban on testimonials for health services.
Almost exactly a year ago, AHPRA published an independent report into doctors who performed cosmetic surgery. The report followed widely reported cases of poor cosmetic surgery practices resulting in terrible patient outcomes.
As a result, the medical regulator created new advertising guidelines and a cosmetic surgery hub for complaints.
At the same time, an agreement was reached between Australia’s health ministers that the title “cosmetic surgeon” – virtually unregulated for 20 years – would become restricted. The amendment Bill is expected to pass and will mean no medical practitioner can call themselves a surgeon without meeting a new accreditation standard.
Yet GPs said the reforms had neglected non-surgical procedures.
There are multiple centres of regulatory enforcement for cosmetic procedures.
While AHPRA and its national boards regulate health practitioners and the advertising of health services, other agencies co-regulate.
The Therapeutic Goods Administration (TGA) reviews and approves all injectable products for market supply. While it has approved many products for cosmetic injection, the TGA identifies such interventions as serious medical procedures.
And while AHPRA deals with the advertising of health services, the TGA will prosecute some advertising breaches while Australian Consumer Law will control others.
Because AHPRA has no real authority to regulate the actions of those who are not health practitioners, the reforms are unlikely to affect those operating outside a medical setting, such as self-injectors or people who go to so-called “Botox parties”.
Still, state laws will make the unauthorised use of schedule 4 drugs an offence in each jurisdiction.
While it is too early to tell if the proposed reforms will improve patient outcomes dramatically, reforms like this play a critical role in setting standards and clarifying patient expectations.![]()
Christopher Rudge, Law lecturer, University of Sydney
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()

Menopausal Hormone Therapy - What Dose of Estrogen is Best?

Cardiovascular Benefits of GLP1s – New Evidence

Oral Contraceptive Pill in Teens

RSV and the Heart

Modified but kept in place
Eliminated entirely without replacement
Maintained as is
Completely replaced with an alternative system
Listen to expert interviews.
Click to open in a new tab
Browse the latest articles from Healthed.
Once you confirm you’ve read this article you can complete a Patient Case Review to earn 0.5 hours CPD in the Reviewing Performance (RP) category.
Select ‘Confirm & learn‘ when you have read this article in its entirety and you will be taken to begin your Patient Case Review.
Menopause and MHT
Multiple sclerosis vs antibody disease
Using SGLT2 to reduce cardiovascular death in T2D
Peripheral arterial disease
