Articles / Vaccination for COVID-19 control and considerations for Australia


writer
Professor of Global Biosecurity, NHMRC L3 Research Fellow, Head, Biosecurity Program, Kirby Institute, UNSW Sydney
0 hours
These are activities that expand general practice knowledge, skills and attitudes, related to your scope of practice.
0 hours
These are activities that require reflection on feedback about your work.
0 hours
These are activities that use your work data to ensure quality results.
These are activities that expand general practice knowledge, skills and attitudes, related to your scope of practice.
These are activities that require reflection on feedback about your work.
These are activities that use your work data to ensure quality results.
Vaccines remain the main prospect for an exit strategy from the COVID-19 pandemic, and may, depending on efficacy, duration of protection and uptake, make herd immunity feasible. If herd immunity is not achievable, SARS-COV-2 will circulate long-term. There are many vaccine candidates in development and choices between vaccines that will vary in efficacy and safety. The efficacy of available vaccines is compared and ranges from 62–95% against symptomatic infection with the G614 variant. Efficacy is reduced against new variants of concern and is uncertain against asymptomatic infection. Some vaccines show a better protective immune response than natural infection. The principles of herd immunity and prerequisites for achieving it, such as vaccine efficacy, duration of protection and coverage, are discussed. The alternative vaccine strategies including mass vaccination, targeted risk or age-based vaccination and ring vaccination, as well as speed of vaccination are reviewed. Finally, the impact of variants of concern on vaccine programs and the logistics of mass vaccination are discussed.
Australia has controlled COVID-19 well, but people live with intermittent restrictions such as social distancing, masks and lockdowns, and health authorities must maintain testing and contact tracing infrastructure. Vaccines remain the main prospect for an exit strategy and may, depending on efficacy, duration of protection and uptake, make herd immunity feasible1. There are over 62 vaccine candidates in clinical development and over 170 in pre-clinical development2, so we will have a diverse choice of vaccines which will vary in efficacy and safety. Some vaccines show a better protective immune response than natural infection, which is promising3.
There are four basic technologies being used for vaccine development:
Vaccine candidates with published phase three trials show efficacy of 62–95% against symptomatic infection, and almost 100% efficacy against severe infection and death for all three vaccines4–6. There is less confidence about this because the trials were not powered for severe infection and death as end-points. Table 1 summarises these results.
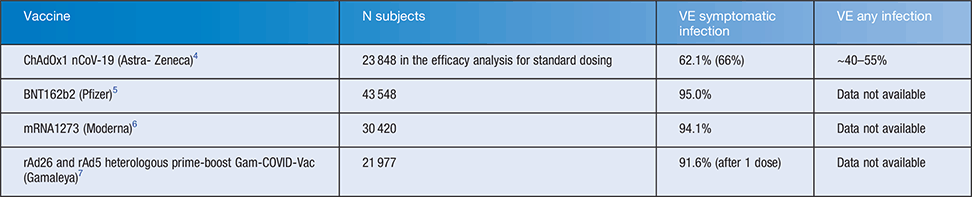 |
The mRNA vaccines5,6 show much higher efficacy against symptomatic infection than the ChAdOx1 nCoV19 vaccine. The ChAdOx1 nCoV19 vaccine has published data on prevention of asymptomatic infection, but measurement of this end-point varied between the trial sites for that vaccine, and analysis was restricted to UK sites. If sites which did not include weekly testing are excluded, the efficacy of this vaccine appears to be between 38–50% against all infection depending on which data are included in the calculation4. There was no significant reduction in asymptomatic infection seen with 2 standard doses of the Astra Zeneca vaccine. However, the efficacy of the Astra Zeneca vaccine was 80% when two standard doses were given at least 12 weeks apart8. Of mRNA vaccines, only the Moderna vaccine trial included data on asymptomatic infection demonstrating a reduction even after a single dose from 39 in placebo to 15 in the vaccine group9. A post-licensure study in Israel showed 92% efficacy of the Pfizer vaccine against all infection10. When vaccine development began in early 2020, the importance of asymptomatic infection was not recognised – now thought to account for up to half of infections11. This may explain the design of clinical trials with symptomatic infection as the primary end-point, when the end-point of interest for herd immunity is prevention of all infection, which includes symptomatic and asymptomatic infection. From an immunological standpoint, symptomatic disease is prevented by good humoral and cell mediated immunity induced by vaccination but immunity at mucosal surfaces in the upper airway may also be important, a still poorly understood mechanism with these current vaccines. The relative importance of systemic and mucosal immunity for prevention of asymptomatic infection is unknown.
Prevention of all infection will prevent transmission, which is necessary for herd immunity and elimination of infection. Elimination and eradication are terms which have specific technical definitions12. Eradication means a pathogen is removed globally, with smallpox being an example. Eradicable pathogens should ideally have no animal host, an effective vaccine and minimal asymptomatic transmission – all of which were met for smallpox. Elimination is the prevention of sustained community transmission in a country or region, and requires herd immunity. Outbreaks may still occur, as we see with measles in Australia, but do not become sustained12. Australia has achieved WHO certified elimination of measles and polio using vaccines13.
SARS-COV-2 is unlikely to be eradicable because of a presumed animal host and substantial asymptomatic transmission. In the absence of a goal for eradication by WHO, countries require technical definitions for elimination with measurable end-points, including time elapsed without community transmission, level of immunity and good surveillance systems12. When elimination and eradication are not possible, “control” is the next best option to reduce the spread14.
The most ambitious and aspirational goal of vaccination programs would be herd immunity and elimination of local transmission. Other goals would be prevention of severe infection and death, which are important in countries experiencing a high incidence of disease. Herd immunity is achieved when vaccination rates are high enough to stop community transmission within a defined population and protects the entire population, whether individuals have been immunized or not, because the number of susceptible individuals is too small for sustained transmission. The required population immunity for herd immunity (H) depends on the basic reproductive number, R0, and can be calculated by the formula:
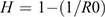
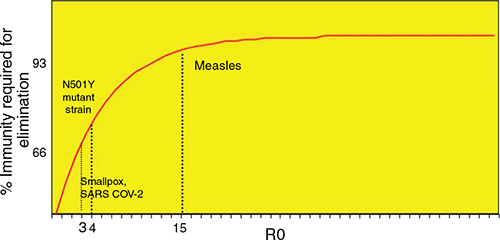
The higher the R, the higher the herd immunity required to control disease, which is a key concept for control of infections through vaccination. Figure 1 shows an elimination graph, and the relationship of R0 to required population immunity for herd immunity, comparing measles (R0 15), smallpox (R0 3), SARS CoV 2 (R0~3)15, and the N501Y mutant strains of SARS-COV-2, which is estimated to be 75% more infectious than the D614G strain, which was dominant globally for most of 202016. Vaccine escape has also been shown against the South African B.1.351 variant, with efficacy of 10% for the Astra Zeneca vaccine17. Elimination of SARS-COV-2 is feasible with a high efficacy vaccine, but if highly transmissible variants become dominant, this will require higher vaccine coverage or even higher efficacy vaccines.
 |
The concept of herd immunity arose from vaccination programs, and yet has been subject to misinformation during the COVID-19 pandemic with theories of ‘natural’ herd immunity being possible by allowing unfettered transmission of SARS-COV-218,19. An unmitigated second wave in Brazil demonstrated this to be false20. Further, the history of epidemic infections such a smallpox, measles and rubella in the pre-vaccine era demonstrates that such infections do not eliminate themselves by ‘natural herd immunity’ – instead, in the absence of vaccines or other control measures, they cause substantial, cycling epidemics which continue indefinitely21. Ongoing cycling epidemics, tempered by intermittent lockdowns and other non-pharmaceutical measures, are also predicted for SARS-COV-2 without vaccines22.
Full or emergency approvals have been granted in many countries for vaccines, mostly those with phase three clinical trial data published such as Pfizer, Moderna and Astra Zeneca vaccines. However, some countries are rolling out vaccines such as CoronaVac (Sinovac) in Indonesia or Covaxin (Bharat Biotech) without published phase three data.
The choice of vaccine strategies for Australia and other countries include:
There may be vaccine shortages initially, so that targeted vaccine is needed initially. Healthcare, aged care and hotel quarantine workers who are at high risk of infection have been prioritised in Australia, followed by groups at significantly higher risk of severe disease, such as older adults23. In Indonesia, young people have been prioritised, as transmission is highest in young adults, so this strategy will impact epidemic growth the most24. Alternatively, a ring vaccination strategy could be used as a dose-sparing strategy. Ring vaccination is the use of vaccines as post-exposure prophylaxis (PEP) to close contacts of cases, who may have been exposed to the virus. Many vaccines, such as measles, hepatitis A and smallpox do have efficacy as PEP25, and are used for outbreak control. Eradication of smallpox was achieved in the last remaining hot-spot, India, using ring vaccination26. The long incubation period of SARS-COV-2 may mean there is efficacy as PEP, but no published data are available yet. We showed that ring vaccination would be the best use of limited doses, if there is efficacy as PEP, and that targeted vaccine strategies would have minimal impact on epidemic growth1. However, herd immunity can only be achieved with mass vaccination.
We do not know yet if herd immunity is achievable. However, given the R0 of SARS-COV-2 in the range of 2–415, it should be feasible with a high efficacy vaccine. If vaccine-induced immunity wanes over time, booster doses may be an option. We do not know the safety or efficacy of giving two different vaccines serially, and this is not always straightforward. For example, conjugate followed by polysaccharide pneumococcal vaccines provide immune boosting, but the reverse causes a diminished response27. Antibody dependant enhancement (ADE)28 is another concern, and was seen with MERS Coronavirus vaccines. The effect of serial administration of different vaccines on the risk of ADE is unknown.
For Australia, aiming for herd immunity requires choosing the highest efficacy vaccines, and planning for at least 70% of the population vaccinated1. A vaccine of less than 70% efficacy against all infection is unlikely to achieve herd immunity, and a vaccine that is very poor at preventing infection will result in ongoing transmission of SARS-COV-2 in the community1. This will place unvaccinated people and people who have vaccine failure such as immunocompromised people, at risk of illness. Unpublished data from Novavax show reduced efficacy for people with HIV infection, for example. The risk of infected people in hotel quarantine is higher now than in March or July 2020, given the pandemic is much worse now. Therefore, the risk of breaches resulting in community outbreaks is now higher. The burden of living with ongoing restrictions and intermittent outbreaks points to the importance of a strategic vaccination policy. Further, the risk of an outbreak of a more transmissible variant is increasing, and such outbreaks will be harder to control than any we have faced previously16.
Vaccine escape is another concern, with unpublished data from Novavax, Johnson and Johnson and Astra Zeneca showing reduced efficacy against variants of concern, particularly the South African and Brazilian variants. The Novavax efficacy in the UK is 95% against G614 but 85% against B117, whilst in South Africa, efficacy against B1351 was 60% (49% when HIV positive subjects included). The Johnson and Johnson single dose vaccine was 66–72% in the UK and US, but 57% against moderate to severe disease in South Africa. However, the Astra Zeneca vaccine was reported to have only 10% efficacy against the South African variant, prompting the South African government to pause plans to roll out that vaccine. On the basis of these developments, the WHO and COVAX have stressed that vaccine manufacturers ‘must be prepared to adjust to the SARS-CoV-2 viral evolution, including potentially providing future booster shots and adapted vaccines, if found to be scientifically necessary’29. The emergence of vaccine escape mutants makes the case for mass vaccination with high efficacy vaccines stronger, as higher population immunity will reduce the selective pressure for further variants to emerge.
For mass vaccination, a slow implementation of vaccine roll out will result in the population living with COVID-19 longer and higher morbidity and mortality1. If vaccination can be delivered at scale rapidly, this will deliver the best outcomes. However, the logistics of rapid delivery are complex and includes vaccination infrastructure, adequate numbers of accredited vaccinators, mass vaccination clinics, a communication and health promotion strategy and a high capacity to deliver large numbers of vaccine doses rapidly.
While many countries have started their COVID-19 vaccination programs, the last time any country conducted mass vaccination was more than 40 years ago, for smallpox. Current EPI programs30 are for infants. In Australia, for example, approximately 4–5 million people a year are vaccinated under the National Immunisation Program, including influenza vaccine for people aged 65 years and over, but mass vaccination would require vaccination of 25 million people. There is no living memory among people in the health workforce on mass vaccination of all age groups. We have excellent knowledge and experience of vaccinating infants and older adults, as well as some high-risk groups. Working age adults are especially challenging, as they are mobile and may not routinely seek healthcare31. The infrastructure and skilled personnel requirements to ensure rapid uptake of mass vaccination, rather than a slow trickle, are significant. We need to be training and accrediting more nurse and pharmacist vaccinators, making plans for public vaccination clinics, ensuring widespread infrastructure for cold chain requirements, especially for vaccines that require deep freezing32, and working proactively on health promotion and communication to ensure high acceptance of vaccination. We could also model the capacity to vaccinate rapidly in each state and territory and expand capacity accordingly. Given the number of good vaccine candidates to come, and the unforeseen circumstances such as those which led to the loss of the University of Queensland vaccine from the Australian planned stockpile, we could diversify our vaccine portfolio. We could also be using vaccines as PEP and collecting data on effectiveness as PEP during outbreaks. Adverse events monitoring is the final component of a vaccine program, as rare side effects may only become apparent post-licensure. Public confidence could be further enhanced with a no-fault vaccine compensation scheme, which is a part of many vaccination programs around the world33.
Raina MacIntyre has consulted for Astra Zeneca and Janssen for COVID-19 vaccines and been on an advisory board on COVID-19 vaccines for Seqirus.
This paper did not receive any specific funding. Raina MacIntyre is funded by a NHMRC Principal Research Fellowship.
Download the PDF version here >>
[1] MacIntyre C.R.et al2020 Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia.medRxiv.2020.12.15.20248278
[2] World Health Organization (2021) Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 17 January 2021).
[3] Poland, G.A. et al. (2020) SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 396, 1595–1606.
| SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates.Crossref | GoogleScholarGoogle Scholar | 33065034PubMed |
[4] Voysey, M. et al. (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111.
| 33306989PubMed |
[5] Polack, F.P. et al. (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615.
| Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine.Crossref | GoogleScholarGoogle Scholar | 33301246PubMed |
[6] Baden, L.R. et al. (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416.
| 33378609PubMed |
[7] Logunov, D.Y. et al. () Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397, 671–681.
[8] Voysey, M. et al. (2021) Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397, 881–891.
| Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials.Crossref | GoogleScholarGoogle Scholar | 33617777PubMed |
[9] Food and Drug Administration (2020) Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. US Department of Health and Human Services, FDA, Silver Spring, MD, USA.
[10] Dagan, N. et al. (2021) BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. , .
| BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting.Crossref | GoogleScholarGoogle Scholar | 33626250PubMed |
[11] Oran, D.P. and Topol, E.J. (2020) Prevalence of asymptomatic SARS-CoV-2 infection. Ann. Intern. Med. 173, 362–367.
| Prevalence of asymptomatic SARS-CoV-2 infection.Crossref | GoogleScholarGoogle Scholar | 32491919PubMed |
[12] Heywood, A.E. and Macintyre, C.R. (2020) Elimination of COVID-19: what would it look like and is it possible? Lancet Infect. Dis. 20, 1005–1007.
| Elimination of COVID-19: what would it look like and is it possible?Crossref | GoogleScholarGoogle Scholar | 32771079PubMed |
[13] Heywood, A.E. et al. (2009) Elimination of endemic measles transmission in Australia. Bull. World Health Organ. 87, 64–71.
| Elimination of endemic measles transmission in Australia.Crossref | GoogleScholarGoogle Scholar | 19197406PubMed |
[14] Dowdle, W.R. (1998) The principles of disease elimination and eradication. Bull. World Health Organ. 76, 22–25.
| 10063669PubMed |
[15] CDC (2020) COVID-19 Pandemic Planning Scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
[16] Leung, K. et al. (2021) Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 26, 2002106.
| 33413740PubMed |
[17] Madhi, S.A. et al. (2021) Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa. medRxiv , .
| Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa.Crossref | GoogleScholarGoogle Scholar |
[18] Aschwanden, C. (2020) The false promise of herd immunity for COVID-19. Nature 587, 26–28.
| The false promise of herd immunity for COVID-19.Crossref | GoogleScholarGoogle Scholar | 33087872PubMed |
[19] Vogel, G. (2020) Sweden’s gamble. Science 370, 159–163.
| Sweden’s gamble.Crossref | GoogleScholarGoogle Scholar | 33033200PubMed |
[20] Buss, L.F. et al. (2021) Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 371, 288.
| Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic.Crossref | GoogleScholarGoogle Scholar | 33293339PubMed |
[21] MacIntyre, C.R. et al. (2019) Evidence of long-distance aerial convection of variola virus and implications for disease control. Viruses 12, 33.
| Evidence of long-distance aerial convection of variola virus and implications for disease control.Crossref | GoogleScholarGoogle Scholar |
[22] Kissler, S.M. et al. (2020) Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368, 860–868.
| Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period.Crossref | GoogleScholarGoogle Scholar | 32291278PubMed |
[23] Australian Government, Department of Health (2020) Preliminary advice from ATAGI on general principles for the COVID-19 vaccination program.
[24] Giubilini, A. et al. (2020) COVID-19 vaccine: vaccinate the young to protect the old? J. Law Biosci. 7, lsaa050.
| COVID-19 vaccine: vaccinate the young to protect the old?Crossref | GoogleScholarGoogle Scholar | 32959006PubMed |
[25] Gallagher, T. and Lipsitch, M. (2019) Postexposure effects of vaccines on infectious diseases. Epidemiol. Rev. 41, 13–27.
| Postexposure effects of vaccines on infectious diseases.Crossref | GoogleScholarGoogle Scholar | 31680134PubMed |
[26] Mohanty, B. et al. (2020) Modelling the impact of a smallpox attack in India and influence of disease control measures. BMJ Open 10, e038480.
| Modelling the impact of a smallpox attack in India and influence of disease control measures.Crossref | GoogleScholarGoogle Scholar | 33318109PubMed |
[27] Greenberg, R.N. et al. (2014) Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine 32, 2364–2374.
| Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age.Crossref | GoogleScholarGoogle Scholar | 24606865PubMed |
[28] Arvin, A.M. et al. (2020) A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584, 353–363.
| A perspective on potential antibody-dependent enhancement of SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar | 32659783PubMed |
[29] WHO (2021) COVAX statement on new variants of SARS-CoV-2. https://www.who.int/news/item/08-02-2021-covax-statement-on-new-variants-of-sars-cov-2
[30] Hoest, C. et al. (2017) Vaccine coverage and adherence to EPI schedules in eight resource poor settings in the MAL-ED cohort study. Vaccine 35, 443–451.
| Vaccine coverage and adherence to EPI schedules in eight resource poor settings in the MAL-ED cohort study.Crossref | GoogleScholarGoogle Scholar | 27998640PubMed |
[31] MacIntyre, C.R. et al. (2016) Equity in disease prevention: vaccines for the older adults – a national workshop, Australia 2014. Vaccine 34, 5463–5469.
| Equity in disease prevention: vaccines for the older adults – a national workshop, Australia 2014.Crossref | GoogleScholarGoogle Scholar |
[32] Callaway, E. (2020) What Pfizer’s landmark COVID vaccine results mean for the pandemic. Nature. , .
| What Pfizer’s landmark COVID vaccine results mean for the pandemic.Crossref | GoogleScholarGoogle Scholar | 33627854PubMed |
[33] Halabi, S. et al. (2020) No-Fault compensation for vaccine injury – the other side of equitable access to Covid-19 vaccines. N. Engl. J. Med. 383, e125.
| No-Fault compensation for vaccine injury – the other side of equitable access to Covid-19 vaccines.Crossref | GoogleScholarGoogle Scholar | 33113309PubMed |

Menopausal Hormone Therapy - What Dose of Estrogen is Best?

Cardiovascular Benefits of GLP1s – New Evidence

Oral Contraceptive Pill in Teens

RSV and the Heart

writer
Professor of Global Biosecurity, NHMRC L3 Research Fellow, Head, Biosecurity Program, Kirby Institute, UNSW Sydney

Modified but kept in place
Eliminated entirely without replacement
Maintained as is
Completely replaced with an alternative system
Listen to expert interviews.
Click to open in a new tab
Browse the latest articles from Healthed.
Once you confirm you’ve read this article you can complete a Patient Case Review to earn 0.5 hours CPD in the Reviewing Performance (RP) category.
Select ‘Confirm & learn‘ when you have read this article in its entirety and you will be taken to begin your Patient Case Review.
Menopause and MHT
Multiple sclerosis vs antibody disease
Using SGLT2 to reduce cardiovascular death in T2D
Peripheral arterial disease
